You are here: Home / Cryo Competence Center Life Science / Biomaterial Biomaterial / 3D-structure on Demand
Model-based freeze-drying
...of collagen scaffolds for cell culture and regenerative medicine
- Free design of the 3D-structure at the PC
- Easy adjustment of stability and degradation characteristics
Competence in cryogenic collagen design
Collagen is a biopolymer of outstanding medical importance. It is used as hemostatic wound dressing, as a drug delivery system for the applications of antibiotics or hormones as well as a biodegradable tissue replacement material for reconstructive surgery and regenerative medicine. Moreover, it plays an important role as carrier structure for cell and tissue culture.
3-dimensional (3D) wound dressings, implantable biomaterials and cell culture scaffolds from collagen and related biomaterials are made are manufactured industrially by freeze-drying. The quality and preparation of the raw materials as well as the cooling step of the freeze-drying process are influencing the structure of the resulting highly porous 3D biopolymer matrix.
We are using these knowledge for a specific adjustment of the matrix structure by means of a model-based ice templating technology (MBIT). By use of our freeze-dryer concept Janus the whole manufacturing process can be performed in one operation.
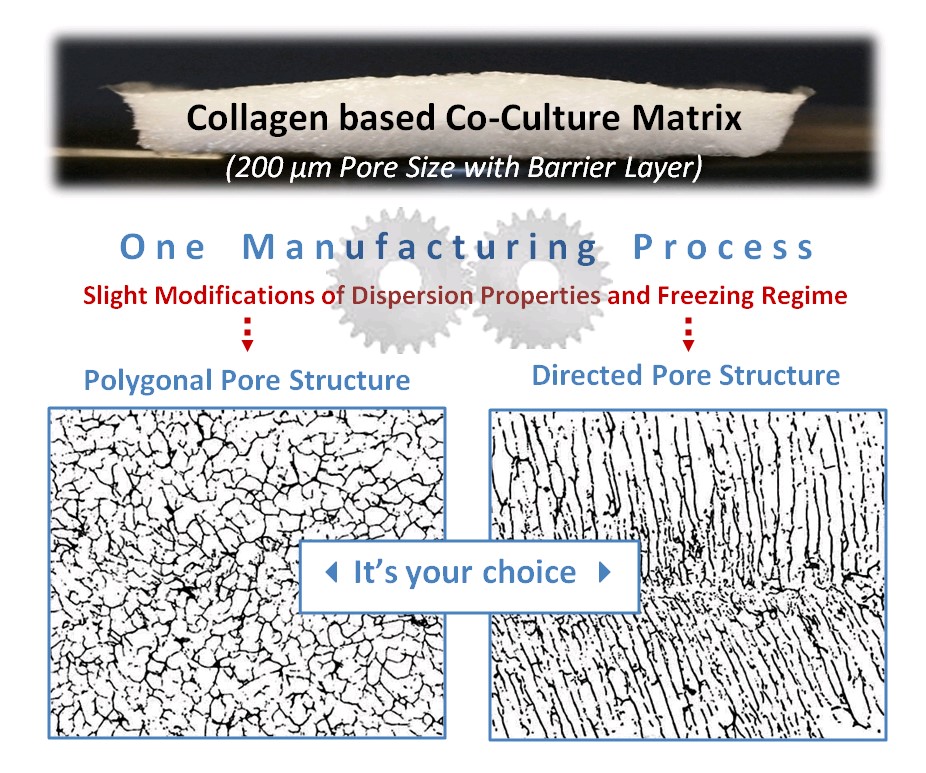
Pore shape, pore size and micro-porosity can be freely adjusted by means of a software-aided process planning. Even bi-layer materials with a barrier against unwanted cell migration can easily be produced this way.
The mechanical strength and degradation properties of the manufactured biomaterial can be adjusted on request by a special thermal treatment in the final step of the lyophilization process.
The broad range of applications for tailor-made, collagen based carrier materials has been confirmed impressively in the field of cell and tissue culture.
Model-based Ice Templating – planning of customized 3D-structures on the PC
Operation of the MBIT procedure
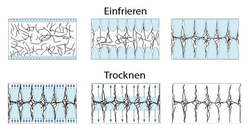
Our patented MBIT technology is based on the principle of a specific cold application to solutions or suspensions for the generation of a defined patterned ice structure. During the freezing process, the dissolved or dispersed substances accumulate at the interfaces of the growing ice crystals. They are compacted to a porous matrix structure with a predefined structural pattern. Subsequently, the frozen water is removed by gentle freeze-drying. In this way, the crystalline ice becomes removed from the matrix structure which remains as a highly porous biomaterial scaffold with the desired structural properties.
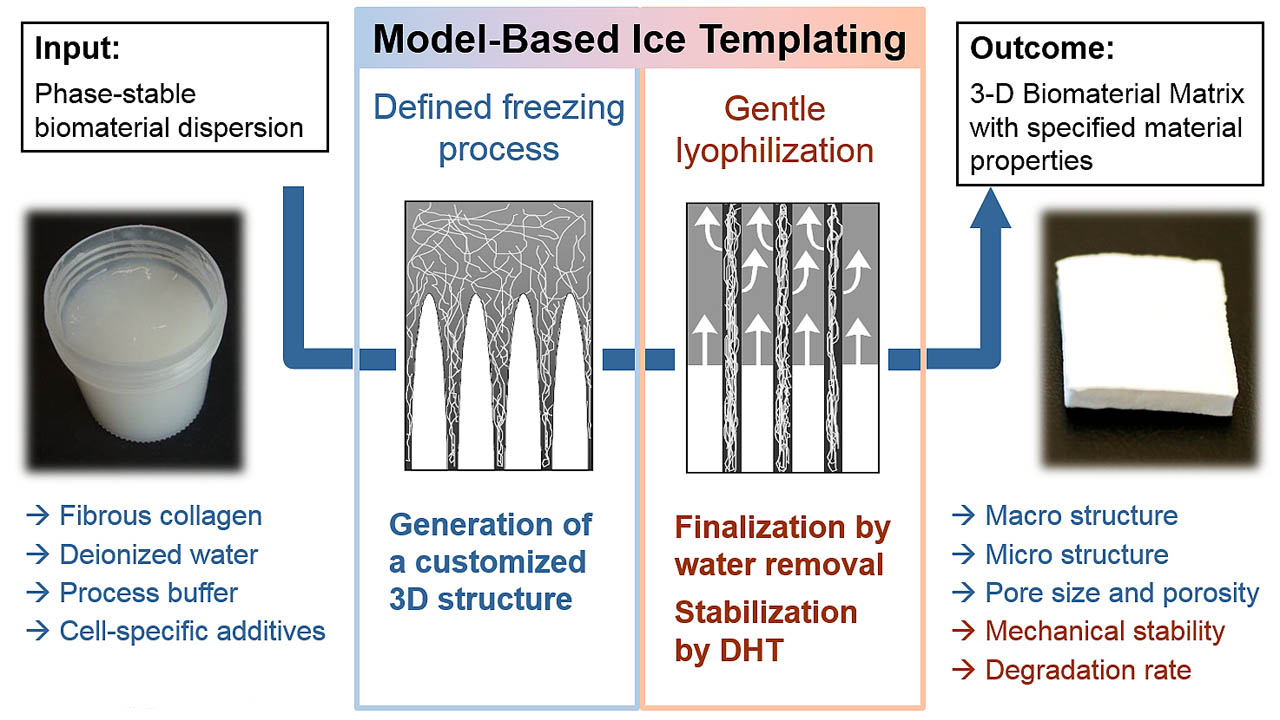
The described technique allows a free choice of the 3D structure, the mechanical strength and the degradation properties of 3D collagen scaffolds by a detailed planning of the production process on the PC. Thus, tailor-made 3D biomaterials for diverse applications can be directly produced without any costly and time consuming research work. The following parameters of the collagen sponge can be influenced specifically in the freeze-drying process:
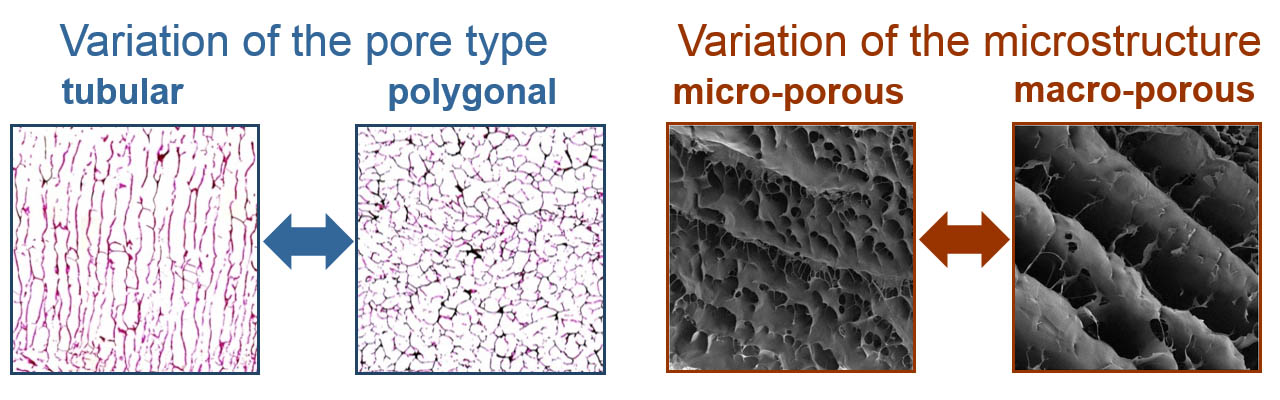
- Choice of either a polygonal or a lamellar-tubular pore structure
- Free variation of the main pore size in the region of 50 µm up to 500 µm
- Free variation of the porosity and micro-porosity
- Generation of a bi-layer structure with a barrier of compacted collagen
- Free choice of the barrier position and the pore sizes in the separated material areas
- Adaption of mechanical strength and degradation rate
In this way, tailor made scaffold for a broad range of applications in the fields of medicine, diagnostics and research can be easily produced. The process control is based on statistical models which allow a free and independent variation of matrix properties within physical limitations. Macro- and microstructure, pore size, porosity, matrix stability and degradation behavior can be adapted for an optimal fit of specific requirements in tissue replacement, 3D cell culture, scientific tissue models or parenteral drug application..
The processing of a biopolymer suspension into a 3D matrix consists of three procedural steps, which can be carried out in one operation:
- Step: Controlles Freezing
- Step: Lyophilization
- Step: Stabilization
Our novel freeze-dryer study Janus combines a double-sided convection cooling system with controlled cooling rates with the common freeze-drying process. Thus, the whole production process can be performed in one operation. Despite of subsequent changes of required product properties there will be no need for further investments in manufacturing technologies, new equipment or further approval procedures. That saves much time and money.
Economic benefits
- Use of conventional freezing and freeze-drying technology
- Flexible product design with an uniform production procedure
- New product designs without development expenses
- Simplified approval of new products by the possibility of retrospective validation
The technology is in particular of interest, when a flexible production of 3-dimensional scaffolds with a tailor-made macro-structure and micro-structure is required, e. g.:
- Rapid diversification of the product portfolio
- Production on customer’s request
- Frequent product changes
Customized composition and 3D-structure
What materials can be processed?
Our core competence is the processing of 3-dimensional scaffolds from fibrous collagen and collagen-based composites with soluble additives and mineral micro-granulates for bone regeneration. But in principle biomaterial matrices with defined structural properties can be processed from all types of biopolymers which are able to form aqueous solutions or phase-stable suspensions. By now, MBIT has been tested for the processing of gelatin, hyaluronates and other GAG as well.
What types of material structure can be generated?
The choice of an appropriate freezing regime enables the production of homogeneous material structures. On the other hand materials with an integrated barrier layer can be produced in a single freeze-drying run without additional process steps. Those materials can be used for cell culture and tissue engineering (co-culture of different cell types) as well as the regenerative medicine (guided tissue regeneration). By the control of the freezing step the structure of the biomaterial can be adapted to individual needs:
Defined macro-structure and micro-structure
The shape of the pores can be varied between a polygonal and a tubular-directed type. This enables the decision between two different base structures which are required by different types of cells for the development of their tissue-specific growth pattern and their tissue-specific metabolic properties.
Porosity and the micro-structure of the biomaterial matrix can also be defined by the controlled biopolymer processing. These parameters are essential for the gas and nutrient supply of the embedded cells. Thus, they enable a targeted control of cell and tissue specific properties. While materials with an open-pored, microporous structure can lead mesenchymal stem cells to form connective tissue, a non-porous structure can force the same cells the same cells to cartilage formation.
Targeted pore size adjustment and implementation of barrier layers
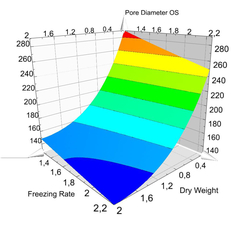
Only by a variation of the freezing rate, scaffolds with pore diameters of less than 100 µm can be generated as easily as macro-porous materials with an average pore size of more than 300 µm. With free parameter choice, MBIT allows a reproducible variation of the mean pore diameter between 50 µm and 500 µm for collagen matrices and collagen-based composites.
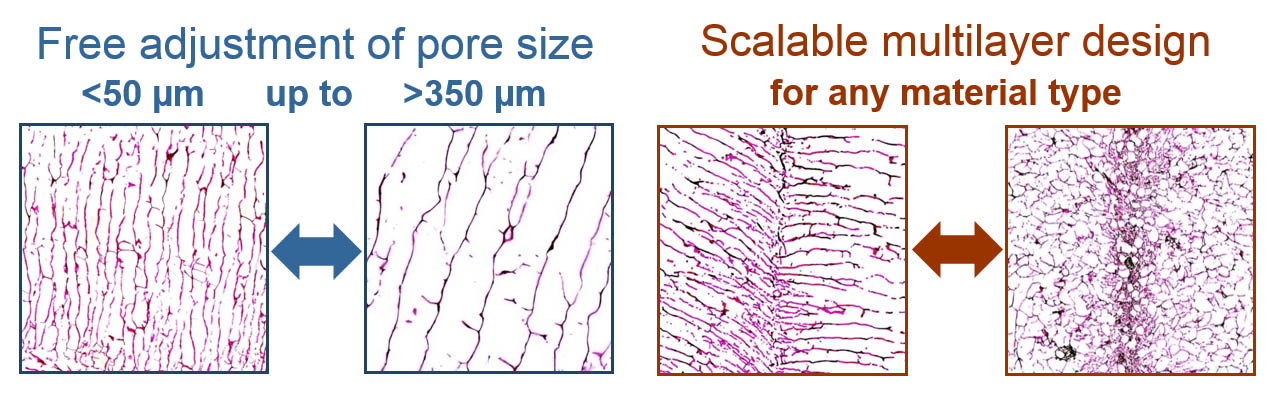
Simultaneously, MBIT enables the generation of a barrier from condensed collagen in the freeze-drying process which is able to regulate or inhibit the cell growth into the opposite region of the scaffold material. The position of the barrier layer inside of the matrix and the pore size ratio of the separated matrix areas can be altered independently within certain limits.
Control of stability and degradation properties
The desired compressive strength and stiffness of the manufactured biomaterials, its resorption rate in the body or its degradation behavior under cell culture conditions often can already be adjusted in the freeze-drying process by a physical cross-linking step. The so-called dehydrothermal treatment (DHT) enables a gentle stabilization of the produced materials without cytotoxic effects. It can be integrated into the manufacturing process without the need of further processing steps. The degradation rate of the biomaterial can be application specific customized by control of the cross-linking temperature.
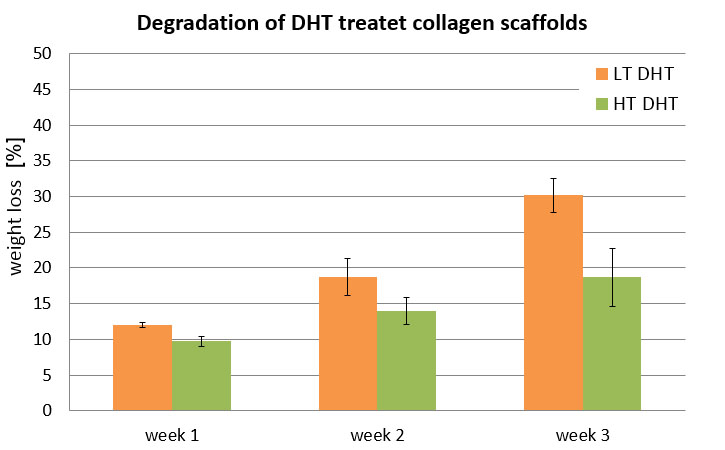
When used as regenerative biomaterial for the treatment of tissue defects, the collagen scaffold is settled by cells from the surrounding tissue and remodeled to endogenous tissue. To provide the space for this natural remodeling process a complete biological degradation of the implant is desired within a few weeks. When the collagen scaffold is used as a matrix for the 3-dimensional cell culture in the lab, long culturing periods are often requested instead. To accommodate the cells during the whole cultivation process, the collagen matrix has to provide a much higher degradation stability. The MBIT technology enables a flexible adjustment of the degradation rate for each application.
Performance of application specific biomaterials under cell culture conditions
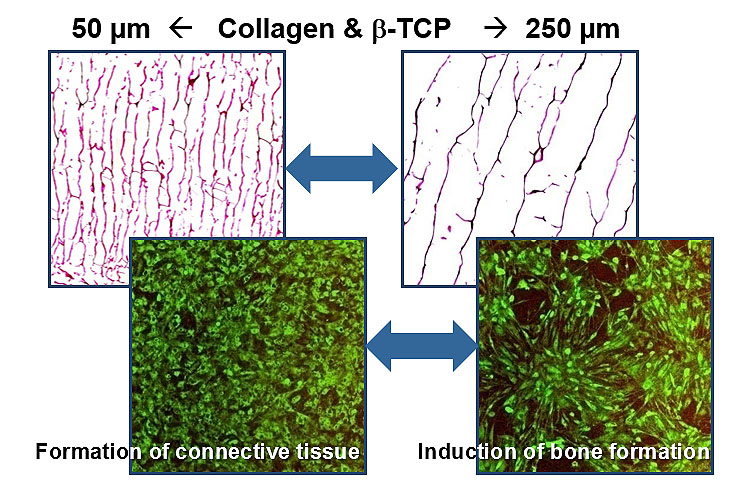
Impact of the pore size onto tissue formation
The pore size of the matrix material is of great significance for the induction of a natural tissue structure in the regenerative medicine as well as the in vitro cell culture. Experiments of an amplified tissue culture of humane bone cells show clearly that a pore size below 100 µm leads to the formation of connective tissue while a mean pore size of 250 µm induces a calcification of the growing tissue structures which leads to bone formation..
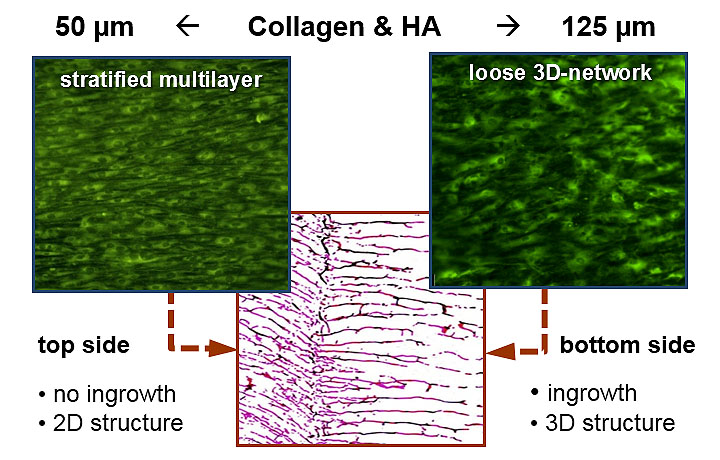
Guided tissue regeneration in bilayer scaffolds
The implementation of migration inhibiting layers of higher material density allows the manufacturing of regenerative biomaterials with barrier function. Those scaffolds can be used for the guided tissue regeneration (GTR) and guided bone regeneration to permit the ingrowth of unwanted cell types into the defect during the tissue regeneration. In the field of tissue engineering bilayer scaffolds offer the possibility of the creation of multi-layer tissue structures like skin or mucosa by a population of the separated matrix areas with different cell types.
The double-sided population of a collagen based bi-layer composite for the tissue engineering of human mucosa with gingival fibroblasts demonstrates the control of tissue formation by the structural properties of the matrix. The superficial cell growth on the small-pored matrix surface leads to the formation of a confluent 2-dimensional surface layer while the same cell type migrates deeply into the large-pored opposite of the scaffold and forms a loose, 3-dimensional network of connective tissue there. As the result, a skin-like tissue structure occurs.