Sie befinden sich hier: Startseite / Kryokompetenzzentrum Life Science / Pharmaceutical Freezing
Analysis and yield optimization in pharmaceutical freezing
Today freezing steps are indispensable for different processing steps in biopharmaceutical production of vaccines, hormones and enzymes to protect the sensitive products against mechanical, enzymatic and bacterial degradation during storage and transport. In-house freezing steps allow a free planning of purification and formulation steps and enable an optimal capacity utilization in the complete downstream process. In addition, the frozen product represents a defined state for quality assessment. Unfortunately, the freezing process itself leads to a significant amount of product damage. Folding and aggregation of sensitive proteins lead to high economic losses.
Monitoring and process analysis
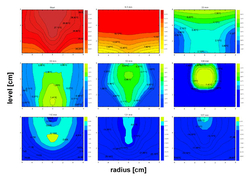
An important step in the understanding of quality problems and product loss in freezing steps is the comprehensive thermal and physico-chemical analytics of the freezing process. With non-contact measurement methods and the systematic sampling and analyses of ice-samples out of the frozen goods, we record the critical concentration- and charge displacement in the freezing process und measure the resulting product quality. By comprehensive investigation of the heat transfer of the packaging material, the heat conduction and convection in the frozen goods and the resulting ice formation kinetics and ice structure, we get an insight into the damaging mechanism and valuable information for the technical optimization of the process. With control of packaging components we ensure your product quality.
Process optimization for the protection of valuable products
Modern biopharmaceuticals, such as recombinant antibodies for cancer treatment, rate among the most valuable industrial goods. Nevertheless in critical but essential freezing steps huge product losses are accepted. This is no longer the case!
We have developed efficient tools for optimization of pharmaceutical freezing processes. Based on time- and cost-efficient stability analysis we provide mathematical models for simulation of protein stability in freezing processes. So product losses for any process conditions can be predicted to optimize the freezing process with less product.